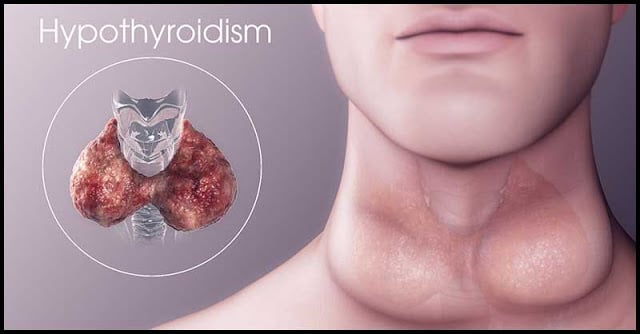
In a recommendation that could “substantially alter prescribing trends,” according to its authors, an international panel of experts concludes that patients with subclinical hypothyroidism should not be routinely offered thyroid hormone replacement therapy.
This is because overwhelming evidence shows no benefit in quality of life or symptoms, which are minimal in many patients and not present at all in one-third of individuals, they outline.
The guideline panel concludes in its recommendation, published online this week in BMJ, that “for adults with subclinical hypothyroidism, thyroid hormones consistently demonstrate no clinically relevant benefits for quality of life or thyroid-related symptoms, including depressive symptoms, fatigue, and body mass index (BMI).”
The guidance — based on findings from a systematic review and meta-analysis of 21 trials with 2192 participants published in November (JAMA. 2018;320:1349-1359) — represents a “strong recommendation” against prescribing thyroid hormones (primarily levothyroxine LT4) in adults with subclinical hypothyroidism, defined as elevated thyroid stimulating hormone (TSH) levels when free T4 (thyroxine) levels are normal.
The new advice was published as part of the BMJ’s rapid recommendations initiative, developed to provide “rapid and trustworthy guidance based on new evidence to help doctors make better decisions with their patients,” according to a BMJ press statement.
Members of the guideline panel, led by chair Mieke Vermandere, MD, Ph.D., of the Academic Centre for General Practice of Public Health and Primary Care, KU Leuven, Belgium, includes clinicians and methodologists, as well as patients themselves, adhering to the standards for trustworthy guidelines using the GRADE approach.
Key Trial Is TRUST Study in Older Adults
Among key findings in the systematic review that prompted the recommendation are those from the TRUST trial, as reported last year by Medscape Medical News — the largest study in the meta-analysis which focused on older patients.
In that trial of 737 adults aged 65 years and older (mean age, 74 years) with a wide variety of comorbidities, therapy with levothyroxine (LT4) showed “no apparent benefits in older persons with subclinical hypothyroidism.”
The panel says the TRUST trial offers highly convincing evidence.
“There was high certainty that there is little to no difference in general quality of life, thyroid-related symptoms, depressive symptoms, fatigue, cognitive function, muscle strength, and BMI,” the panel writes.
“The results are consistent across these outcomes, which strengthens our confidence that there really is a lack of benefit.”
They note also, however, that the evidence against treating subclinical hypothyroidism extends beyond older patients, applying to a broad range of adults with the condition.
More Than Half Have Normalization of TSH Levels Without Treatment
Subclinical hypothyroidism is reported to affect about 5% of the adult population and 10-15% of the elderly; however, the definition can vary. About 90% of patients with subclinical hypothyroidism have TSH levels of 4-10 mlU/L, but a slight increase may be normal in older people.
When symptoms are present, they can include fatigue, muscle cramps, sensitivity to cold, sluggish thinking, and depression; however, the panel notes that 20% to 25% of people with normal thyroid levels report one or two of these symptoms.
And in the majority of cases, the issue resolves itself. About 62% of people with TSH levels of 4-10 mlU/L experience normalization of thyroid levels within 5 years without any treatment, the report notes. The risk of overt hypothyroidism emerging from the subclinical forms of the condition ranges between 2% and 5% per year.
And although observational data have suggested a link between subclinical hypothyroidism and an increased risk of coronary heart disease, the associations have not been seen with TSH levels of 5-10 mIU/L.
Prescriptions Have Soared Beyond Prevalence
Under current guidelines, thyroid hormones are recommended when TSH levels are above 10 mlU/L, and for patients with lower TSH values who are symptomatic, young, or have other indications for treatment.
Yet prescriptions of thyroid hormone replacement therapy for subclinical hypothyroidism have sharply risen. In 2015, levothyroxine was among the most prescribed drugs in the United States.
One study in the systematic review showed a doubling of prescriptions in the UK in 1996-2006, and increases were reported in a Norwegian population as well — despite no corresponding increases in the incidence of subclinical hypothyroidism.
And another study (JAMA Intern Med. 2014;174:32-39) also included in the review showed as many as a third of patients were offered levothyroxine after just a single TSH test, despite the evidence that TSH levels fluctuate and often return to normal on their own.
The panel notes that “with such an approach it is difficult to separate real from placebo effects,” and the JAMA study also showed that once patients start levothyroxine therapy, most remain on the drug for several years.
Avoid Burden of Lifelong Therapy, Monitor, but Make Exceptions
Although there is uncertainty about the potential harms of the possibly unnecessary use of levothyroxine, the burden of lifelong management itself should also be factored into the equation, the panel notes.
Instead of prescribing treatment with thyroid hormone, “clinicians should monitor the progression or resolution of the thyroid dysfunction in these adults,” they advise.
There are exceptions. The recommendation specifically does not apply to women trying to become pregnant and those with TSH levels above 20 mlU/L, and it “may not” apply to patients with severe symptoms or young adults aged 30 years and younger, the panel notes.
“For younger people (under 30 years of age) and for patients with unusually high TSH levels (> 20 mIU/L with normal T4 levels) the evidence remains more indirect, although this concerns only a small minority of patients,” they write.
The BMJ rapid recommendation is a collaborative effort with the MAGIC group, and a full version of the recommendation, including decision aids, can be accessed using the MAGICapp.
The panel members have reported no relevant financial relationships.
BMJ 2019. Published online May 14, 2019. Full text
Important Notice: This article is originally published by Nancy A. Melville in www.medscape.com where all credits are due.