The HPV Vaccine: A Double-Edged Sword? (Part 1)
The human papillomavirus (HPV) vaccine has been recommended by doctors as the main measure to take against cervical cancer. Many people, including doctors, think there is no risk since there are no recognized cases with proven association with the vaccine.
But is this true? Does the HPV vaccine really deliver the stellar protection promised or is it a double-edged sword with risks that outweigh the benefits?
With nearly two decades of experience as a medical doctor with a doctorate in infectious disease, I have been dedicated to extensive research, allowing me to acquire profound insights into diverse viruses and vaccines. I have been actively engaged in numerous clinical research projects, acquiring a wealth of knowledge in assessing the efficacy and safety of pharmaceutical products. My expertise extends beyond laboratory experiments and encompasses a comprehensive understanding of their tangible impact on individuals.
Since taking the sacred Hippocratic oath 32 years ago, which solemnly emphasizes the principle: “First, do no harm,” I have felt a profound sense of responsibility. This responsibility compels me to write about the HPV vaccine, which carries significant implications for the lives of young individuals.
For those who are considering the HPV vaccine, here are my thoughts and recommendations.
HPV Vaccines and CDC Recommendations
There have been three HPV vaccines—the quadrivalent HPV vaccine (Gardasil, 4vHPV, approved in June 2006), the bivalent HPV vaccine (Cervarix, 2vHPV, approved in October 2009), and the 9-valent HPV vaccine (Gardasil 9, 9vHPV, approved in December 2014)—all licensed by the U.S. Food and Drug Administration (FDA).
The HPV virus has over 200 strains. There are 14 high-risk HPV strains that are associated with cervical cancer: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
Since Cervarix was withdrawn from the U.S. market in 2016 due to “very low market demand,” the HPV vaccines we focus on in this series are Gardasil and Gardasil 9.
According to the CDC, “HPV vaccine is recommended for routine vaccination at age 11 or 12 years. HPV vaccines can be given starting at age 9.” The HPV vaccination is given as a series of either two or three doses, depending on age at initial vaccination.
Safety Assessment of Vaccines Is Stricter Than for Drugs
When we talk about vaccines, people normally do a risk-benefit analysis. The bar set for the safety of a vaccine is much higher than that for a therapeutic drug.
Drugs are used for patients who need medicine to live their lives. Preventive vaccines are primarily designed for healthy people to protect them from contracting a contagious disease.
Because vaccines are given to a healthy population, they normally undergo rigorous safety and efficacy studies in the pre- and post-licensure stages. The safety assessment of a vaccine is supposed to be very strict and thorough before approval.
A drug is prescribed to treat a patient with a medical condition, such as heart disease. A patient may choose to take the drug to improve their symptoms or reduce their risk of death.
The doctor will assess whether the benefits of the drug outweigh the risks of possible side effects. When a patient is suffering from a medical condition, the benefits of a drug often outweigh the risks.
Since a vaccine is administered to a healthy person to protect them from a potential future contagious disease, the risks must be made completely clear. They must be well-informed about the short- and long-term safety and tolerability of a vaccine that has the potential to alter their life by compromising their healthy immune system.
Most importantly, a preventive vaccine should not be associated with or contribute to a death or disability risk known as a “severe adverse event” (SAE).
- Results in death.
- Is life-threatening.
- Requires inpatient hospitalization or extension of existing hospitalization.
- Results in persistent or significant disability/incapacity.
- Is a congenital anomaly/birth defect.
Although there is no legal requirement for “zero death tolerance” related to a preventive vaccine, continuing to administer the vaccine when death appears to be associated, clearly violates the Hippocratic oath.
In April 2016, the Philippines began a dengue disease vaccination (Dengvaxia) program for 9-year-old children. The program involved around 875,000 children each given at least one dose; around 400,000 with two doses; and 350,000 receiving all three doses.
As of September 2018, 19 deaths of children were confirmed to be linked with the vaccination. In February 2019, the license was revoked in the Philippines.
A prominent pediatrician and medical researcher in the Philippines was indicted over the failed introduction of Dengvaxia.
After thorough research on the safety of the HPV vaccine and based on my causal analysis, there have been reported SAEs, including deaths, that are associated with the HPV vaccine.
Deaths of Healthy Girls After HPV Vaccine, Reported by FDA and CDC
During my career, I have also worked in the field of medical safety for pharmaceutical companies. My role was to determine whether a causal relationship existed between a particular drug with an SAE. I have thoroughly evaluated hundreds of cases with serious adverse events.
Based on my work in this area, I am confident to attribute the causal assessment of the following events to the HPV vaccine. An assessment of risks is essential to making an informed decision about whether to take the HPV vaccine.
A comprehensive analysis was conducted based on SAEs reported to the Vaccine Adverse Event Reporting System associated with the administration of qHPV vaccine, Gardasil, in females aged 9 to 26 years. The study was published in the top-rank medical journal JAMA and it is authored by the FDA and CDC.
Within only two years of the Gardasil vaccine availability, there were 32 deaths with a mean age of 18. The median time to death was 14.5 days, and the mean time to death was 47 days. Among these 32 death cases, six were cardiac-related (four arrhythmias and two cases of myocarditis), and three were related to pulmonary embolism.
It’s crucial to emphasize that this group of young, healthy girls, with relatively clean medical backgrounds, free of preexisting health conditions to attribute to their death, died within a median time of 14.5 days following qHPV vaccination.
Based on the standard industry criteria (pdf) for the assessment of causality of a drug with an adverse event, these death events had a plausible time relationship to the vaccine administration, and they cannot be explained by other preexisting medical conditions in these young girls. The causal relationship with the HPV vaccine, if not highly likely, should be considered as at least “probable/likely/plausible” and not excluded.
If I were to regard these deaths as “not related,” it would be an irresponsible judgment on my part and a violation of my oath as a medical doctor.
Others may argue that these deaths are unrelated, with no evidence recognizing an association with the HPV vaccine.
However, just because a causal relationship hasn’t been fully recognized doesn’t mean that a relationship is nonexistent.
Cochrane Review Reported Higher Death Rates in HPV Vaccine Group
A Cochrane review (pdf) reported more deaths in the HPV vaccine groups than in the comparator groups. The death rate was significantly increased by 2.36 times in women older than 25 years of age.
This data was hidden in the “Table Summary of Findings 3” on page 10. Page 36 states, “When all the deaths among mid-adult women enrolled in the three trials are pooled, a higher case fatality rate was observed among those who received HPV vaccine compared to those who received placebo.”
The report also stated, “The higher number of deaths in the vaccine arms among mid-adult women may be a chance occurrence since there was no pattern either in the causes of death, or in the timing of the occurrence of death (the period between vaccine administration and date of death). In the study reports, none of the deaths were deemed to be related to vaccination.”
From a standard safety analysis, in each study, every death case has to provide a thorough narrative regarding the causality analysis of the role of the HPV vaccine. Age, gender, time from vaccination to death, and the cause of death, must be provided in detail.
The causality of these death cases remains questionable. Further due diligence is warranted and an investigation on the cause of death should be conducted.
Deaths Due to Fatal Autoimmune Vasculitis
Autoimmune vasculitis is an autoimmune-related inflammation of a blood vessel caused when the body’s immune system attacks a blood vessel by mistake.
In 2012, a peer-reviewed research article published in Pharmaceutical Regulatory Affairs: Open Access reported research on two deaths after HPV vaccination (pdf).
The research was conducted to determine if serious autoimmune and neurological adverse drug reactions following HPV vaccination were causal or merely coincidental and to validate the protocol used for assessing causality in the vaccination-suspected serious adverse neurological outcomes.
IHC is a way to stain targeted substances, such as proteins, at a microscopic level by using the principle of antigen and antibody binding (“immuno-“) within the tissues (“histo-“) and then using a coloring method (“chemical”) to make the tissue visible under the microscope.
In the following cases, the HPV viral protein was stained for identification. Brain sections were also stained for antibodies recognizing HPV-16L1 and HPV-18L1 antigens, which are present in the Gardasil vaccine.
The details of the medical history of the two cases and IHC results are cited below:
Case 1: Death of 19-year-old Girl
The report stated:
“A 19-year-old female (pdf) without a relevant medical history and taking no drugs expired in her sleep, approximately 6 months after her third and final qHPV vaccine booster and following exacerbation of initial vaccination-related symptoms. She had last been seen alive by her parents the previous evening.
“Her symptoms started after the first qHPV injection when she developed warts on her hand that persisted throughout the vaccination period. In addition, she suffered from unexplained fatigue, muscle weakness, tachycardia, chest pain, tingling in extremities, irritability, mental confusion and periods of amnesia (memory lapses).”
According to the report, the normal autopsy was unremarkable and failed to determine the exact cause of death of this girl.
Case 2: Death of 14-year-old Girl
The report stated:
“A 14-year-old female (pdf) with a previous history of migraines and oral contraceptive use developed more severe migraines, speech problems, dizziness, weakness, inability to walk, depressed consciousness, confusion, amnesia and vomiting 14 days after receiving her first qHPV vaccine injection. These symptoms gradually resolved.
“However, 15 days after her second qHPV vaccine booster she was found unconscious in her bathtub by her mother 30 minutes after she had entered the bathroom to have a shower. Emergency help was summoned and arrived quickly. Resuscitation efforts were attempted. The paramedic noted that the patient was found without a pulse. Upon arrival at the hospital and approximately 30 minutes later, the patient suffered cardiac arrest. Resuscitation was terminated approximately 40 minutes later and the patient was pronounced dead.”
Similar to Case 1, the standard autopsy failed to identify a precise cause of death:
“Nonetheless, autopsy revealed cerebral edema and cerebellar herniation indicative of a focally disrupted blood-brain barrier. … There were however acidophilic changes of the Purkinje cells in the cerebellum with vacuolation of the overlying molecular layer.”
These findings suggest a local breakdown of the blood-brain barrier, a crucial structure that usually prevents harmful substances from entering the brain from the bloodstream. Moreover, it suggests some damage or degeneration has occurred in the cerebellum, specifically involving the Purkinje cells and the molecular layer, essential for movement and coordination.
The IHC analyses (pdf) in both cases provided the following cause of death:
“The results from our IHC examinations of brain tissue specimens from two young women who died following vaccination with the qHPV vaccine Gardasil showed strong evidence of an autoimmune vasculitis triggered by the cross-reactive HPV-16L1 antibodies binding to the wall of cerebral blood vessels (Figures 1 and 2). In addition, there was clear evidence of the presence of HPV-16L1 particles within the cerebral vasculature with some HPV-16L1 particles adhering to the blood vessel walls (Figures 1C, 2C, and 2D).”
In Figure 1, Case 1 below, the vascular walls of two small blood vessels show positive immunoreactivity for virus-like particles (VLPs) of the major capsid (L1) protein of HPV-16.
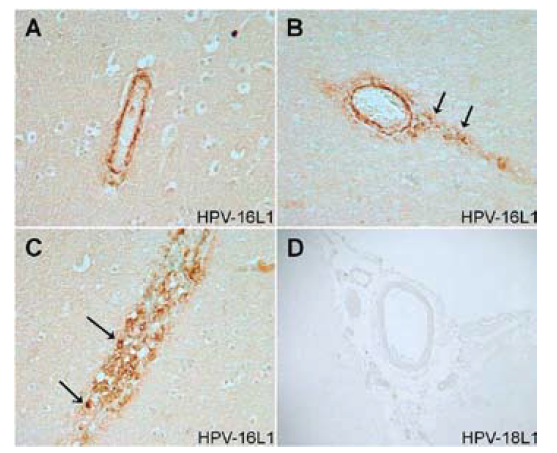
Smoking Gun: HPV Vaccine Component–16L1
The authors concluded that HPV vaccines containing HPV-16L1 antigens pose an inherent risk for triggering potentially fatal autoimmune vasculitis.
The major capsid protein, L1, is the main membrane protein of HPV. 16L1 is the relevant protein belonging to HPV type 16.
In both cases of death, the IHC analysis showed evidence of autoimmune vasculitis—inflammation of the blood vessels—potentially triggered by the cross-reactive HPV-16L1 antibodies binding to the wall of cerebral blood vessels in all examined brain samples.
This is a significant concern, as it demonstrates that HPV vaccine-derived immune complexes are capable of penetrating the blood-brain barrier.
Researchers from the French National Institute of Health and Medical Research, INSERM, have published a paper with evidence that aluminum adjuvant nanoparticles taken up by white blood cells after injection travel to draining lymph nodes, then cross the blood-brain barrier, and eventually accumulate in the brain where they can cause significant immune-inflammatory adverse reactions.
Thus, the presence of HPV-16L1 particles in the brain vessels from both young women vaccinated with Gardasil may be explained by a “Trojan horse” mechanism dependent on circulating macrophages by which these particles absorbed the aluminum adjuvant to gain access to brain tissue. In other words, this only happened because the aluminum adjuvant opened the door by going through the lymph nodes, crossing the blood-brain barrier, and letting the 16L1 particles come in.
To summarize: The HPV VLPs in Gardasil, particularly HPV-16L1, are adsorbed on the aluminum adjuvant and then taken up by monocytes after injection. The VLPs first travel to draining lymph nodes, which allows them to cross the blood-brain barrier and eventually accumulate in the brain where they can cause significant immune-inflammatory adverse reactions, as evidenced by the deaths of both girls.
What Does This Mean?
It appears that in some cases Gardasil may be the triggering factor in fatal autoimmune/neurological events. Physicians should be aware of this association.
Cerebral vasculitis is a serious disease that typically results in fatal outcomes when undiagnosed and left untreated.
The fact that many of the symptoms reported to vaccine safety surveillance databases following HPV vaccination are indicative of cerebral vasculitis, but are unrecognized as such (e.g., intense persistent migraines, syncope, seizures, tremors and tingling, myalgia, locomotor abnormalities, psychotic symptoms, and cognitive deficits), is a serious concern in light of the present findings.
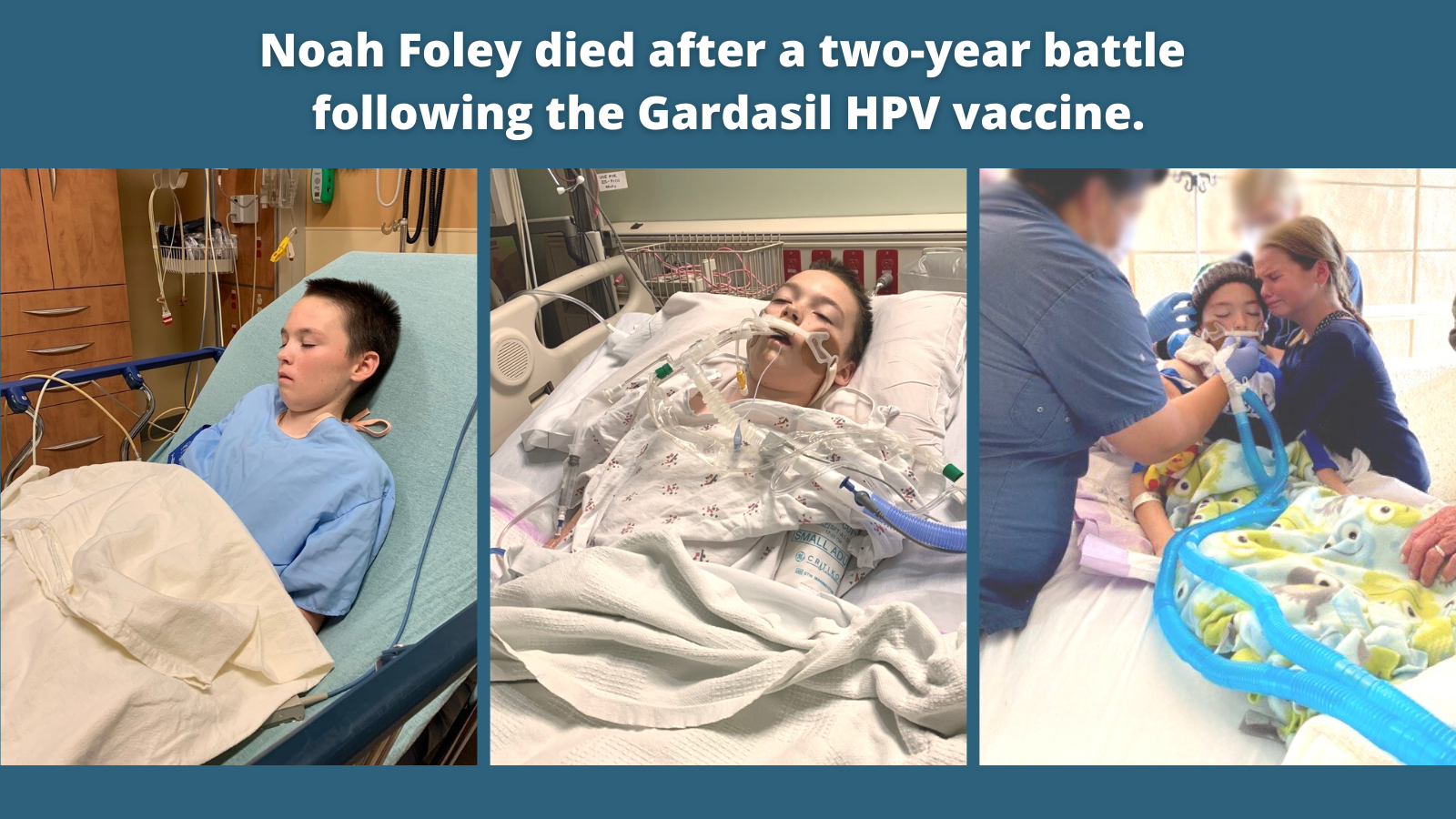
Noah Foley, 13 Years, Died of Autoimmune Encephalitis After Gardasil
“Noah Tate Foley received his first and only Gardasil injection on May 7, 2018, just two days after his 11th birthday. Noah enjoyed hunting and fishing with his dad, playing games with his younger sister, building Legos, and playing his drum set. He loved school and was active in his church. Most of all, Noah loved his family and treasured the times they spent together.
“Prior to the Gardasil shot, Noah had no autoimmune diseases and no autonomic issues. He was extremely healthy, having received a clean bill of health during a medical check-up. Roughly two weeks after the Gardasil shot, Noah experienced fevers that reached as high as 102.9 degrees. His symptoms continued and one week later, his blood was checked to rule out Mononucleosis or other causes for the ongoing fevers. Testing revealed no ‘cause’ for his fevers, which came and went throughout the summer of 2018.
“On October 10, 2018, Noah went to the emergency room at Duke University Medical Center in Durham, North Carolina. After examination and blood tests, Noah’s mother, Kelli Foley, was informed that her son’s inflammatory markers were elevated, possibly due to a viral infection. Noah was then referred to the Duke University Infectious Disease department, where blood work revealed that Noah’s white blood cell count had tripled in two weeks.
“For months, Noah endured countless doctor visits and testing, including a CT scan and biopsy of a swollen lymph node. Kelli Foley recalled the 35 days between the discovery of the swollen lymph node and a report that ruled out cancer as ‘long and torturous.’ Still, the family had no answers to the underlying cause of Noah’s health issues.
“On May 7, 2019, Noah had an appointment for weight loss where the records state:
‘Over the past year, [Noah] has had a rough year. He was in his usual state of good health per Mother until he went for his 11-year-old vaccine and well child check-up. After that he continues to have fevers and fatigue. He has been seen by multiple specialists over the past 7 months – starting in October 2018. He has had one lymph node removed from his neck as well as CT scan (neck/abdomen) and MRI to evaluate what inflammatory process may be occurring. He has continued to have fatigue and not feel like himself. It has been noted that over the past year he has lost 20lb despite continued good vertical height growth and continued to eat fairly well …’
“Noah’s weight was 69 pounds, his BMI was in the 4th percentile at 14.79, and his inflammatory markers remained elevated.
“At a May 21, 2019 pediatric gastroenterology consultation, the assessment discussed an ‘autoimmune or inflammatory process.’
“On the afternoon of September 29, 2020, Noah’s left leg went numb. While his mother rushed him to the emergency room, Noah’s face and tongue went numb. By the time he arrived at the ER, Noah vomited, and by 6:00 p.m., he was completely non-responsive. Noah was transported to Duke University Medical Hospital, where his condition rapidly declined.
“On September 30, 2020, Noah was almost completely brain dead. On October 8, 2020, Noah passed away four hours after his breathing tube was removed. He was 13 years old.
“According to the Foley’s lawsuit allegations, Noah died of encephalitis caused by an autoimmune/autoinflammatory dysregulation process, which was caused-in-fact by the Gardasil vaccination received in 2018.
“‘Our faith is very strong, which is why I know that despite the pain our family continues to feel in Noah’s absence, we won’t let his death be in vain.’ Kelli Foley said. ‘We will fight for him in getting justice against Merck for what they did to him.’”
The Foleys have said that if they had been warned about Gardasil’s potential to cause serious adverse health effects, they would not have given consent for their son to receive the vaccine.
Christina Tarsell, 21 Years, Died of Arrhythmia After Gardasil
According to Christina’s documented legal case:
“On June 23, 2008, a 21-year-old student at Bard College died suddenly and unexpectedly in bed at the New York home she shared with other students. Christina Tarsell was young and healthy; a multi-sport athlete who played softball in high school and tennis at Bard.
“Medical examiners listed Christina’s cause of death as undetermined. Unable to live without answers to Christina’s unexplainable death, the Tarsell family pursued an extensive investigation involving world-class experts in immunology, cardiology and electrophysiology.
“The doctors evaluating Christina Tarsell’s death determined that she died of an arrhythmia induced by an autoimmune response to Gardasil, an HPV vaccine that she had received only days before her death.
“Merck & Co. advertised the Gardasil vaccine to young women as an effective tool to prevent cervical cancer. The Centers for Disease Control and Prevention (CDC) recommended that girls and women receive three shots of Gardasil.
“Days after receiving her first Gardasil shot, Christina Tarsell suffered from arrhythmia. She experienced the same problem again days after her second Gardasil shot. Ms. Tarsell died after her third Gardasil injection.
“Emily Tarsell, Christina’s mother, decided to pursue legal action over her daughter’s wrongful death against the Department of Health and Human Services (HHS). As a covered vaccine, victims of Gardasil injuries cannot file a Gardasil lawsuit against Merck in civil court before first filing a claim with the Vaccine Injury Compensation Program (VICP).
“On April 2, 2018, after the Tarsell family fighting many years for justice, a special master in the VICP ruled that Christina had died from a heart arrhythmia caused by Gardasil. The decision stated that Tarsell’s legal team had presented ‘preponderant evidence of a logical sequence of cause and effect, connecting the HPV vaccination to the ensuing arrhythmia.’
“Emily Tarsell is one of many Gardasil victims to file a claim in the VICP. As of January 2023, 798 Gardasil claims have been filed in the VICP for 21 deaths and 777 injuries that occurred after HPV vaccination (pdf). Of those, 167 resulted in compensation for the victims.”
In 2020, Emily Tarsell, a therapist and Christina’s mother, wrote in her legal testimony (pdf): “Experts determined that she died from the vaccine and after 8 years of litigation in the vaccine court, the government conceded that Gardasil caused her death.”
Potential Causes of Death Requiring Investigation
Autoimmunity
In the case of Noah Foley and the two deaths reported in the journal, the common mechanism for injury was autoimmunity.
A lack of pattern observation (signal detection) is one of the main reasons cited for rejecting the causal role of Gardasil in these cases. However, autoimmune disorders are dependent on the interplay between genetic and environmental factors and have a very diversified clinical profile. Depending on the attacked organ, genetic background, and predisposition factors, patients may have a striking diversity of clinical features.
A 2019 study published in Pathobiology observed that the proteins used in HPV overlap with many human proteins.
As early as 2011, the researcher suggested a potential mechanism behind cardiac problems associated with Gardasil.
The paper revealed that there are 34 peptides composed of five amino acids, known as pentamers, in the virus’s protective shell or “capsid” protein, which are identical to sequences found in human proteins.
This means that when people receive the HPV vaccine, the generated antibodies might not only attack HPV, but can also bind to our own proteins, including titin, changing their structure. Altering the structure of titin can cause severe cardiac problems.
We will provide a more detailed mechanistic analysis of the evidence of injuries related to Gardasil in the next articles.
Blood Clots
The 2009 JAMA study previously cited found a disproportionately high (four- to sixfold) increase in venous thromboembolic events after HPV vaccination.
The FDA and CDC authors stated: “The PRR for 6- to 17-year-olds was 4.8 (P = .04). The PRR for 18- to 29-year-olds was 6.7 (P = .006). Both of these age groups met the screening criteria for signal detection.”
The proportional reporting ratio (PRR) is the proportion of spontaneous reports for a given drug that are linked to a specific adverse outcome, divided by the corresponding proportion for all or several other drugs. A PRR greater than one could indicate that the adverse event is caused by the drug and is considered a “side effect.”
This is a very disturbing signal as pulmonary embolism is a potentially life-threatening condition that may explain the death cases after the HPV vaccine.
Gardasil: A Vaccine With Inadequate Safety Assessment
Gardasil’s package insert lacks adequate information related to preclinical safety assessment. An assessment report (pdf) by the European Medicines Agency states: “Toxicology studies of AAHS alone were not performed because this adjuvant has been used before in several other Merck vaccines and has an established safety profile.”
As a product to be given to healthy people, Gardasil vaccines should follow rigorous preclinical and clinical safety assessments before approval for human use. However, the FDA and CDC reported 32 death cases of young girls with a suspected causal role of Gardasil, and a Cochrane review reported higher death rates in the HPV vaccine group.
In addition, a peer-reviewed article reported detailed medical records, autopsy, and immunological staining reports revealing Gardasil vaccine-associated fatal autoimmune vasculitis as the potential cause of death. There are also at least two documented U.S. cases associated with Gardasil currently in litigation.
Next: We will provide a collection of documented evidence for serious injuries associated with Gardasil. Their existence is undeniable.
References:
- The Hippocratic Oath. National Institute of Health. https://www.nlm.nih.gov/hmd/greek/greek_oath.html#:~:text=I%20will%20use%20those%20dietary,pessary%20to%20cause%20an%20abortion
- Human Papillomavirus (HPV) Vaccination: What Everyone Should Know. https://www.cdc.gov/vaccines/vpd/hpv/public/index.html
- Burd, E. M. (2003). Human Papillomavirus and Cervical Cancer. Clinical Microbiology Reviews, 16(1), 1-17. https://doi.org/10.1128/CMR.16.1.1-17.2003
-
HPV and Cancer. National Cancer Institute. https://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-and-cancer#:~:text=High%2Drisk%20HPVs%20can%20cause,for%20most%20HPV%2Drelated%20cancers
- GSK exits U.S. market with its HPV vaccine Cervarix. Eric Sagonowsky Oct 21, 2016 11:52am. https://www.fiercepharma.com/pharma/gsk-exits-u-s-market-its-hpv-vaccine-cervarix
- Ensuring the Safety of Vaccines in the United States. 2011. FDA. https://www.fda.gov/files/vaccines,%20blood%20&%20biologics/published/Ensuring-the-Safety-of-Vaccines-in-the-United-States.pdf
- ICH HARMONISED TRIPARTITE GUIDELINE CLINICAL SAFETY DATA MANAGEMENT: DEFINITIONS AND STANDARDS FOR EXPEDITED REPORTING E2A Current Step 4 version dated 27 October 1994. https://database.ich.org/sites/default/files/E2A_Guideline.pdf
- Magal, P., Seydi, O., Webb, G., & Wu, Y. (2021). A Model of Vaccination for Dengue in the Philippines 2016–2018. Frontiers in Applied Mathematics and Statistics, 7, 760259. https://doi.org/10.3389/fams.2021.760259
- The dengue vaccine dilemma. Dec 16, 2019. Pharmaceutical Technology. https://www.pharmaceutical-technology.com/features/dangvaxia-philippines/
-
Dengue vaccine fiasco leads to criminal charges for researcher in the Philippines Rose Capeding could face years in prison for her role in clinical trials of Dengvaxia. 24 APR 2019. By Fatima Arkin. https://www.science.org/content/article/dengue-vaccine-fiasco-leads-criminal-charges-researcher-philippines
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure Safety Surveillance for Quadrivalent Human Papillomavirus Recombinant Vaccine. JAMA. 2009;302(7):750–757. doi:10.1001/jama.2009.1201 https://jamanetwork.com/journals/jama/fullarticle/184421
- The use of the WHO-UMC system for standardized case causality assessment. https://who-umc.org/media/164200/who-umc-causality-assessment_new-logo.pdf
- Arbyn, M., Xu, L., Simoens, C., & Martin-Hirsch, P. P. (2018). Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. The Cochrane database of systematic reviews, 5(5), CD009069. https://doi.org/10.1002/14651858.CD009069.pub3
- Ooi, J. D., Deng, J., & S. De Souza, A. W. (2021). Editorial: Autoimmune Vasculitis – Advances in Pathogenesis and Therapies. Frontiers in Immunology, 12. https://doi.org/10.3389/fimmu.2021.720257
- Tomljenovic L, Shaw CA (2012) Death after Quadrivalent Human Papillomavirus (HPV) Vaccination: Causal or Coincidental? Pharmaceut Reg Affairs S12:001. doi:10.4172/2167-7689.S12-001 https://www.semanticscholar.org/paper/Death-after-Quadrivalent-Human-Papillomavirus-(HPV)-Tomljenovic-Shaw/c3c61bef84afe05aeb6a297abd0eb7151876b84e
-
Buck, C. B., Day, P. M., & Trus, B. L. (2013). The Papillomavirus Major Capsid Protein L1. Virology, 445, 169. https://doi.org/10.1016/j.virol.2013.05.038
- Gherardi, R. K., & Authier, J. (2012). Macrophagic myofasciitis: Characterization and pathophysiology. Lupus, 21(2), 184. https://doi.org/10.1177/0961203311429557
- 13-Year-Old Noah Foley’s Untimely Death After Gardasil. https://www.wisnerbaum.com/prescription-drugs/gardasil-lawsuit/gardasil-injury-cases/
- Christina Tarsell. https://www.wisnerbaum.com/prescription-drugs/gardasil-lawsuit/gardasil-deaths/
- Emily Tarsell, LCPC, LCPAT. Opposed to HB 87 / SB 135. https://mgaleg.maryland.gov/cmte_testimony/2020/hgo/2579_02122020_113516-311.pdf
- Kanduc D. (2011). Potential cross-reactivity between HPV16 L1 protein and sudden death-associated antigens. Journal of experimental therapeutics & oncology, 9(2), 159–165. https://pubmed.ncbi.nlm.nih.gov/21699023/
-
Pisetsky, D. S. (2023). Pathogenesis of autoimmune disease. Nature Reviews Nephrology, 1-16. https://doi.org/10.1038/s41581-023-00720-1
- Tonino, P., Kiss, B., Strom, J., Methawasin, M., Smith, J. E., Kolb, J., Labeit, S., & Granzier, H. (2017). The giant protein titin regulates the length of the striated muscle thick filament. Nature Communications, 8(1), 1-11. https://doi.org/10.1038/s41467-017-01144-9
- Campuzano, O., Riuró, H., Allegue, C., Coll, M., Mates, J., Picó, F., Iglesias, A., & Brugada, R. (2015). Rare Titin (TTN) Variants in Diseases Associated with Sudden Cardiac Death. International Journal of Molecular Sciences, 16(10), 25773-25787. https://doi.org/10.3390/ijms161025773
- Campuzano, O., Sanchez-Molero, O., Mademont-Soler, I., Riuró, H., Allegue, C., Coll, M., Pérez-Serra, A., Mates, J., Picó, F., Iglesias, A., & Brugada, R. (2015). Rare Titin (TTN) Variants in Diseases Associated with Sudden Cardiac Death. International Journal of Molecular Sciences, 16(10), 25773-25787. https://doi.org/10.3390/ijms161025773
- Rothman, K. J., Lanes, S., & Sacks, S. T. (2004). The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and drug safety, 13(8), 519–523. https://doi.org/10.1002/pds.1001
- EMA/CHMP/76591/2015 Committee for Medicinal Products for Human Use (CHMP). Assessment report Gardasil 9 International non-proprietary name: human papillomavirus 9-valent vaccine (RECOMBINANT, ADSORBED) Procedure No. EMEA/H/C/003852/0000. 26 March 2015. https://www.ema.europa.eu/en/documents/assessment-report/gardasil-9-epar-public-assessment-report_en.pdf
Important Notice: This article was originally published at www.theepochtimes.com by Yuhong Dong M.D., PH.D. where all credits are due.
Disclaimer
The watching, interacting, and participation of any kind with anything on this page does not constitute or initiate a doctor-patient relationship with Dr. Farrah™. None of the statements here have been evaluated by the Food and Drug Administration (FDA). The products of Dr. Farrah™ are not intended to diagnose, treat, cure, or prevent any disease. The information being provided should only be considered for education and entertainment purposes only. If you feel that anything you see or hear may be of value to you on this page or on any other medium of any kind associated with, showing, or quoting anything relating to Dr. Farrah™ in any way at any time, you are encouraged to and agree to consult with a licensed healthcare professional in your area to discuss it. If you feel that you’re having a healthcare emergency, seek medical attention immediately. The views expressed here are simply either the views and opinions of Dr. Farrah™ or others appearing and are protected under the first amendment.
Dr. Farrah™ is a highly experienced Licensed Medical Doctor certified in evidence-based clinical nutrition, not some enthusiast, formulator, or medium promoting the wild and unrestrained use of nutrition products for health issues without clinical experience and scientific evidence of therapeutic benefit. Dr. Farrah™ has personally and keenly studied everything she recommends, and more importantly, she’s closely observed the reactions and results in a clinical setting countless times over the course of her career involving the treatment of over 150,000 patients.
Dr. Farrah™ promotes evidence-based natural approaches to health, which means integrating her individual scientific and clinical expertise with the best available external clinical evidence from systematic research. By individual clinical expertise, I refer to the proficiency and judgment that individual clinicians acquire through clinical experience and clinical practice.
Dr. Farrah™ does not make any representation or warranties with respect to the accuracy, applicability, fitness, or completeness of any multimedia content provided. Dr. Farrah™ does not warrant the performance, effectiveness, or applicability of any sites listed, linked, or referenced to, in, or by any multimedia content.
To be clear, the multimedia content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health providers with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read or seen in any website, video, image, or media of any kind. Dr. Farrah™ hereby disclaims any and all liability to any party for any direct, indirect, implied, punitive, special, incidental, or other consequential damages arising directly or indirectly from any use of the content, which is provided as is, and without warranties.